The effect of various factors on metal corrosion

General classification of factors affecting metal corrosion
The set of factors affecting metal corrosion can be divided into two main categories of environmental factors and intrinsic factors of the metal.
- Environmental factors
- Intrinsic factors of the metal
The most important environmental factors affecting metal corrosion are:
1_ Temperature
In general, increasing temperature increases the speed of chemical reactions. Therefore, with increasing temperature, the corrosion rate also increases. Usually, for every 10 degrees Celsius of temperature, the reaction rate doubles, of course, this rule has many exceptions, and cannot accurately explain the effect of increasing temperature on corrosion reactions.
For example, the corrosion rate of drinking water increases fourfold from 15.6 degrees Celsius to 80 degrees Celsius. In a closed system, the corrosion rate also increases continuously with increasing temperature. The reason for this is that oxygen under pressure cannot be removed from the environment and corrosion continues until all dissolved oxygen is consumed.
2_ Fluid velocity
The destruction of the initial protective layers formed on the metal surface due to high flow velocity can cause corrosion to intensify. The effect of mechanical abrasion alone is not discussed, but increasing the fluid flow rate causes the destruction of thin layers formed on metal surfaces and the bare metal surface will be exposed to corrosion again, therefore, examining the fluid flow velocity in corrosive environments should be considered.
The movement of the corrosive solution causes oxygen to reach the metal surface more easily, which is why the corrosion rate of metals in contact with moving solutions is usually higher than that of metals in contact with stationary solutions.
3_ Stress
When materials and parts are subjected to tensile stresses in corrosive environments, they undergo environmental cracking and are destroyed due to corrosion. In general, stresses such as: residual stresses from heat treatment, welding, cold work, or external stresses imposed on metals in the vicinity of corrosive environments cause corrosion to intensify.
4_ Effect of oxygen concentration and oxidants
One of the important corrosion factors in corrosive aqueous environments is the amount of oxygen dissolved in the aqueous solution, so that with the increase of oxygen and oxides, the corrosion rate also increases continuously.
The effect of oxygen and pH also has a great influence on the corrosion rate of metals. The presence of a difference in air blowing (aeration) on surfaces adjacent to electrolytes causes the creation of anodic and cathodic zones.
For example, in the case of solutions that are in contact with oxygen in the air, sometimes the oxygen in the air does not reach the entire surface of the metal equally, this condition causes the concentration of dissolved oxygen to increase in one area of the solution and decrease in another area. In such solutions, differential pressure cells are formed and will cause corrosion.
5_ Time
The progress and spread of corrosion effects and damage usually increases with time. In some cases, there is a linear relationship between them, of course, there are also conditions where the corrosion rate decreases with time.
The most important intrinsic factors affecting metal corrosion are:
1_ Material type
An alloy is a material that is a mixture of two or more metals (in some cases, non-metals) and can also contain a limited amount of non-metallic elements.
In general, all non-uniformities, whether chemical, metallurgical or mechanical, can be considered a good source for the initiation of corrosion. Therefore, as a general rule for corrosion control, metals with greater uniformity should be used and non-uniformity should be avoided. Some metallurgical factors and characteristics of the metal are of great importance.
Crystal structure, grain boundaries, mechanical properties of metals and alloys, dislocations, specific crystal growth directions, casting methods, heat treatment and chemical composition of alloys are important and effective factors on corrosion.
2_ Metal surface conditions
The onset and rate of corrosion on polished and clean metal surfaces are very different and variable compared to rough and rough surfaces or surfaces that have surface layers. A smooth and polished surface has a lower corrosion rate than a rough surface, because the protective surface layer or rutile on a polished surface is more continuous and uniform than the same layer on rough surfaces. Among them, surfaces that have been recently roughened, such as sandblasted surfaces, are most prone to corrosion.
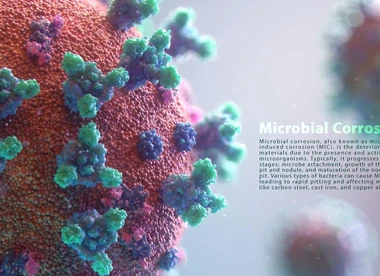
3_ Biological effects
The effect of bacteria and microorganisms on the corrosion rate of various materials has been studied and many experiments have been conducted, and their damage and dangers have been identified. Sometimes, very large numbers of bacteria are exposed, and their damage and dangers have been identified. Sometimes, bacteria and microorganisms create concentration difference cells by creating layers or barriers on the surface of metals, and in some cases, they lead to metal destruction by absorbing hydrogen from the surface of the metal (hydrogen is considered a resistant factor in corrosion cells). Among these bacteria, sulfate-reducing bacteria (SRB) can be mentioned, which produce iron sulfides in areas close to the cathode points and ultimately lead to increased corrosion.
share :












Submit your opinion
Your email address will not be published.